- Record: found
- Abstract: found
- Article: found
Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs)
Read this article at
Abstract
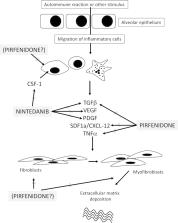
Interstitial lung diseases (ILDs), which can arise from a broad spectrum of distinct aetiologies, can manifest as a pulmonary complication of an underlying autoimmune and connective tissue disease (CTD-ILD), such as rheumatoid arthritis-ILD and systemic sclerosis (SSc-ILD). Patients with clinically distinct ILDs, whether CTD-related or not, can exhibit a pattern of common clinical disease behaviour (declining lung function, worsening respiratory symptoms and higher mortality), attributable to progressive fibrosis in the lungs. In recent years, the tyrosine kinase inhibitor nintedanib has demonstrated efficacy and safety in idiopathic pulmonary fibrosis (IPF), SSc-ILD and a broad range of other fibrosing ILDs with a progressive phenotype, including those associated with CTDs. Data from phase II studies also suggest that pirfenidone, which has a different—yet largely unknown—mechanism of action, may also have activity in other fibrosing ILDs with a progressive phenotype, in addition to its known efficacy in IPF. Collectively, these studies add weight to the hypothesis that, irrespective of the original clinical diagnosis of ILD, a progressive fibrosing phenotype may arise from common, underlying pathophysiological mechanisms of fibrosis involving pathways associated with the targets of nintedanib and, potentially, pirfenidone. However, despite the early proof of concept provided by these clinical studies, very little is known about the mechanistic commonalities and differences between ILDs with a progressive phenotype. In this review, we explore the biological and genetic mechanisms that drive fibrosis, and identify the missing evidence needed to provide the rationale for further studies that use the progressive phenotype as a target population.
Related collections
Most cited references119
- Record: found
- Abstract: found
- Article: not found
Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis.
- Record: found
- Abstract: found
- Article: not found
An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias.
- Record: found
- Abstract: found
- Article: not found