- Record: found
- Abstract: found
- Article: found
Atomic Force Microscopy for Protein Detection and Their Physicoсhemical Characterization
Read this article at
Abstract
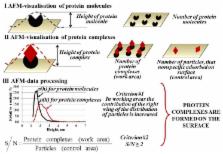
This review is focused on the atomic force microscopy (AFM) capabilities to study the properties of protein biomolecules and to detect the proteins in solution. The possibilities of application of a wide range of measuring techniques and modes for visualization of proteins, determination of their stoichiometric characteristics and physicochemical properties, are analyzed. Particular attention is paid to the use of AFM as a molecular detector for detection of proteins in solutions at low concentrations, and also for determination of functional properties of single biomolecules, including the activity of individual molecules of enzymes. Prospects for the development of AFM in combination with other methods for studying biomacromolecules are discussed.
Related collections
Most cited references138
- Record: found
- Abstract: not found
- Article: not found
Atomic Force Microscope
- Record: found
- Abstract: found
- Article: not found
Single Molecule Force Spectroscopy on Polysaccharides by Atomic Force Microscopy
- Record: found
- Abstract: found
- Article: not found