- Record: found
- Abstract: found
- Article: found
Co-implantation of magnesium and zinc ions into titanium regulates the behaviors of human gingival fibroblasts
Read this article at
Abstract
Soft tissue sealing around implants acts as a barrier between the alveolar bone and oral environment, protecting implants from the invasion of bacteria or external stimuli. In this work, magnesium (Mg) and zinc (Zn) are introduced into titanium by plasma immersed ion implantation technology, and their effects on the behaviors of human gingival fibroblasts (HGFs) as well as the underlying mechanisms are investigated. Surface characterization confirms Mg and Zn exist on the surface in metallic and oxidized states. Contact angle test suggests that surface wettability of titanium changes after ion implantation and thus influences protein adsorption of surfaces. In vitro studies disclose that HGFs on Mg ion-implanted samples exhibit better adhesion and migration while cells on Zn ion-implanted samples have higher proliferation rate and amounts. The results of immunofluorescence staining and real-time reverse-transcriptase polymerase chain reaction (RT-PCR) suggest that Mg mainly regulates the motility and adhesion of HGFs through activating the MAPK signal pathway whereas Zn influences HGFs proliferation by triggering the TGF-β signal pathway. The synergistic effect of Mg and Zn ions ensure that HGFs cultured on co-implanted samples possessed both high proliferation rate and motility, which are critical to soft tissue sealing of implants.
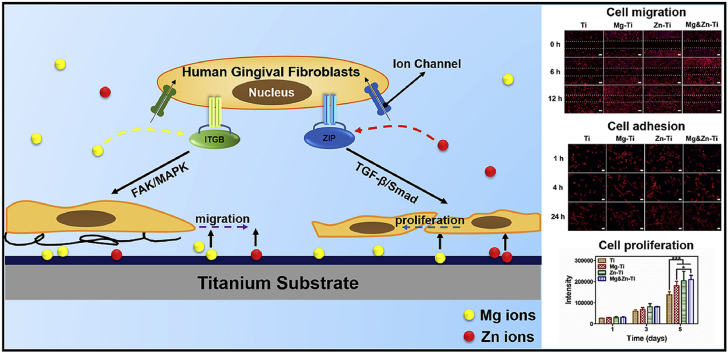
Graphical abstract
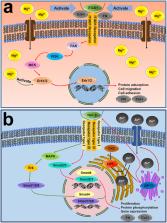
Released Mg ions from the surface spur the expression of ITGB1 and influence HGFs adhesion and migration. Zn ions regulate HGFs proliferation through stimulating Zn transporters gene. Their impacts on HGFs probably involve MAPK and TGF-β signal pathways. Mg and Zn ions implantation improve cell migration and proliferation of HGFs, two important cell behaviors for soft tissue regeneration, respectively.
Highlights
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties.
- Record: found
- Abstract: not found
- Article: not found
Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties
- Record: found
- Abstract: found
- Article: not found