- Record: found
- Abstract: found
- Article: found
Senescent cell turnover slows with age providing an explanation for the Gompertz law
Read this article at
Abstract
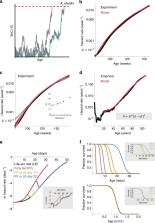
A causal factor in mammalian aging is the accumulation of senescent cells (SnCs). SnCs cause chronic inflammation, and removing SnCs decelerates aging in mice. Despite their importance, turnover rates of SnCs are unknown, and their connection to aging dynamics is unclear. Here we use longitudinal SnC measurements and induction experiments to show that SnCs turn over rapidly in young mice, with a half-life of days, but slow their own removal rate to a half-life of weeks in old mice. This leads to a critical-slowing-down that generates persistent SnC fluctuations. We further demonstrate that a mathematical model, in which death occurs when fluctuating SnCs cross a threshold, quantitatively recapitulates the Gompertz law of mortality in mice and humans. The model can go beyond SnCs to explain the effects of lifespan-modulating interventions in Drosophila and C. elegans, including scaling of survival-curves and rapid effects of dietary shifts on mortality.
Abstract
One of the underlying causes of aging is the accumulation of senescent cells, but their turnover rates and dynamics during ageing are unknown. Here the authors measure and model senescent cell production and removal and explore implications for mortality.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas.
- Record: found
- Abstract: found
- Article: not found
Living on a break: cellular senescence as a DNA-damage response.
- Record: found
- Abstract: found
- Article: not found