- Record: found
- Abstract: found
- Article: found
P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: A review
Read this article at
Graphical abstract
Abstract
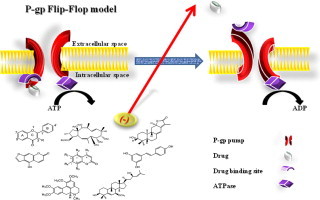
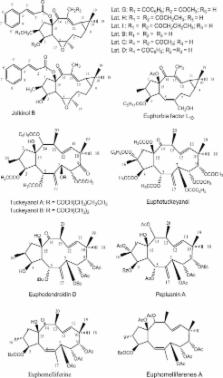
Resistance of solid tumors to treatment is significantly attributed to pharmacokinetic reasons at both cellular and multi-cellular levels. Anticancer agent must be bio-available at the site of action in a cytotoxic concentration to exert its proposed activity. P-glycoprotein (P-gp) is a member of the ATP-dependent membrane transport proteins; it is known to pump substrates out of cells in ATP-dependent mechanism. The over-expression of P-gp in tumor cells reduces the intracellular drug concentrations, which decreases the cytotoxicity of a broad spectrum of antitumor drugs. Accordingly, P-gp inhibitors/blockers are potential enhancer for the cellular bioavailability of several clinically important anticancer drugs such as, anthracyclines, taxanes, vinca alkaloids, and podophyllotoxins. Besides several chemically synthesized P-gp inhibitors/blockers, some naturally occurring compounds and plant extracts were reported for their modulation of multidrug resistance; however, this review will focus only on major classes of naturally occurring inhibitors viz., flavonoids, coumarins, terpenoids, alkaloids and saponins.
Related collections
Most cited references126
- Record: found
- Abstract: found
- Article: not found
Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells.
- Record: found
- Abstract: found
- Article: not found
Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues.
- Record: found
- Abstract: found
- Article: not found