- Record: found
- Abstract: found
- Article: found
TREM2 Deficiency Aggravates NLRP3 Inflammasome Activation and Pyroptosis in MPTP-Induced Parkinson’s Disease Mice and LPS-Induced BV2 Cells
Read this article at
Abstract
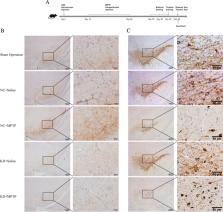
Microglia-mediated neuroinflammation plays a crucial role in the pathogenesis of Parkinson’s disease (PD). Triggering receptor expressed on myeloid cells 2 (TREM2) confers strong neuroprotective effects in PD by regulating the phenotype of microglia. Recent studies suggest that TREM2 regulates high glucose-induced microglial inflammation through the NLRP3 signaling pathway. This study aimed to investigate the effect of TREM2 on NLRP3 inflammasome activation and neuroinflammation in PD. Mice were injected with AAV-TREM2-shRNA into both sides of the substantia nigra using a stereotactic injection method, followed by intraperitoneal injection of MPTP to establish chronic PD mouse model. Behavioral assessments including the pole test and rotarod test were conducted to evaluate the effects of TREM2 deficiency on MPTP-induced motor dysfunction. Immunohistochemistry of TREM2 and tyrosine hydroxylase (TH), immunohistochemistry and immunofluorescence Iba1, Western blot of NLRP3 inflammasome and its downstream inflammatory factors IL-1β and IL-18, and the key pyroptosis factors GSDMD and GSDMD-N were performed to explore the effect of TREM2 on NLRP3 inflammasome and neuroinflammation. In an in vitro experiment, lentivirus was used to interfere with the expression of TREM2 in BV2 microglia, and then lipopolysaccharide (LPS) and adenopterin nucleoside triphosphate (ATP) were used to stimulate inflammation to construct a cellular inflammation model. The expression differences of NLRP3 inflammasome and its components were detected by qPCR and Western blot. In vivo, TREM2 knockdown aggravated the loss of dopaminergic neuron and the decline of motor function. After TREM2 knockdown, the number of activated microglia was significantly increased, and the expression of cleaved caspase-1, NLRP3 inflammasome, IL-1β, GSDMD, and GSDMD-N was increased. In vitro, TREM2 knockdown aggravated the inflammatory response of BV2 cells stimulated by LPS and promoted the activation of NLRP3 inflammasome through the NF-κB pathway. In addition, TREM2 knockdown also promoted the expression of TLR4/MyD88, an upstream factor of the NF-κB pathway. Our vivo and vitro data showed that TREM2 knockdown promoted NLRP3 inflammasome activation and downstream inflammatory response, promoted pyroptosis, and aggravated dopaminergic neuron loss. TREM2 acts as an anti-inflammatory in PD through the TLR4/MyD88/NF-κB pathway, which extends previous findings and supports the notion that TREM2 ameliorates neuroinflammation in PD.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death.
- Record: found
- Abstract: found
- Article: not found