- Record: found
- Abstract: found
- Article: found
Effects of corticosterone on mild auditory fear conditioning and extinction; role of sex and training paradigm
Read this article at
Abstract
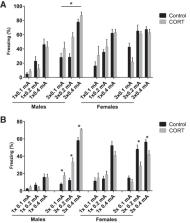
Multiple lines of evidence suggest that glucocorticoid hormones enhance memory consolidation of fearful events. However, most of these studies involve male individuals. Since anxiety, fear, and fear-associated disorders present differently in male and female subjects we investigated in mice whether male and female mice perform differently in a mild, auditory fear conditioning task and tested the modulatory role of glucocorticoid hormones. Using an auditory fear conditioning paradigm with different footshock intensities (0.1, 0.2, and 0.4 mA) and frequencies (1× or 3×), we find that intraperitoneal injections with corticosterone (2 mg/kg) immediately after training, altered freezing behavior when repeated footshocks were applied, and that the direction of the effects were opposite in male and female mice. Effects were independent of footshock intensity. In male mice, corticosterone consistently increased freezing behavior in response to the tone, whereas in female mice, corticosterone reduced freezing behavior 24 h after training. These effects were not related to the phase of the oestrous cycle. In addition, corticosterone enhanced extinction learning for all tones, in both male and female mice. These results emphasize that glucocorticoid hormones influence memory consolidation and retrieval, and underscore sex-specific effects of glucocorticoid hormones in modulating conditioned fear responses.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
Stress and the brain: from adaptation to disease.
- Record: found
- Abstract: found
- Article: not found
Sex bias in neuroscience and biomedical research.
- Record: found
- Abstract: found
- Article: not found