- Record: found
- Abstract: found
- Article: found
Prospective Detection of Early Lung Cancer in Patients With COPD in Regular Care by Electronic Nose Analysis of Exhaled Breath
Read this article at
Abstract
Background
Patients with COPD are at high risk of lung cancer developing, but no validated predictive biomarkers have been reported to identify these patients. Molecular profiling of exhaled breath by electronic nose (eNose) technology may qualify for early detection of lung cancer in patients with COPD.
Research Question
Can eNose technology be used for prospective detection of early lung cancer in patients with COPD?
Study Design and Methods
BreathCloud is a real-world multicenter prospective follow-up study using diagnostic and monitoring visits in day-to-day clinical care of patients with a standardized diagnosis of asthma, COPD, or lung cancer. Breath profiles were collected at inclusion in duplicate by a metal-oxide semiconductor eNose positioned at the rear end of a pneumotachograph (SpiroNose; Breathomix). All patients with COPD were managed according to standard clinical care, and the incidence of clinically diagnosed lung cancer was prospectively monitored for 2 years. Data analysis involved advanced signal processing, ambient air correction, and statistics based on principal component (PC) analysis, linear discriminant analysis, and receiver operating characteristic analysis.
Results
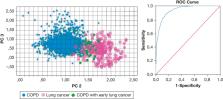
Exhaled breath data from 682 patients with COPD and 211 patients with lung cancer were available. Thirty-seven patients with COPD (5.4%) demonstrated clinically manifest lung cancer within 2 years after inclusion. Principal components 1, 2, and 3 were significantly different between patients with COPD and those with lung cancer in both training and validation sets with areas under the receiver operating characteristic curve of 0.89 (95% CI, 0.83-0.95) and 0.86 (95% CI, 0.81-0.89). The same three PCs showed significant differences ( P < .01) at baseline between patients with COPD who did and did not subsequently demonstrate lung cancer within 2 years, with a cross-validation value of 87% and an area under the receiver operating characteristic curve of 0.90 (95% CI, 0.84-0.95).
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Reduced lung-cancer mortality with low-dose computed tomographic screening.
- Record: found
- Abstract: not found
- Article: not found
Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial
- Record: found
- Abstract: found
- Article: not found