- Record: found
- Abstract: found
- Article: not found
Eosinophils: changing perspectives in health and disease
Read this article at
Key Points
-
Eosinophils have been traditionally perceived as terminally differentiated cytotoxic effector cells. Recent studies have provided a more sophisticated understanding of eosinophil effector functions and a more nuanced view of their contributions to the pathogenesis of various diseases, including asthma and respiratory allergies, eosinophilic gastrointestinal diseases, hypereosinophilic syndromes and parasitic infection.
-
Eosinophils are granulocytes that develop in the bone marrow from pluripotent progenitors in response to cytokines, such as interleukin-5 (IL-5), IL-3 and granulocyte–macrophage colony-stimulating factor (GM-CSF). Mature eosinophils are released into the peripheral blood and enter tissues in response to cooperative signalling between IL-5 and eotaxin family chemokines.
-
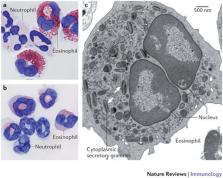
Eosinophils in peripheral blood and tissues are uniquely identified by their bilobed nuclei, their large specific granules that store cytokines, cationic proteins and enzymes, and their expression of the IL-5 receptor and CC-chemokine receptor 3 (CCR3). In addition, the receptors sialic acid-binding immunoglobulin-like lectin 8 (SIGLEC-8) and SIGLEC-F are expressed by human and mouse eosinophils, respectively.
-
IL-5 has a central and profound role in all aspects of eosinophil development, activation and survival. IL-5 is produced by T helper 2 (T H2) cells, and more recently the contributions of the epithelium-derived innate cytokines thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 in promoting eosinophilia via the induction of IL-5 have also been recognized.
-
Although eosinophil responses are influenced by cytokines produced by T cells, eosinophils in turn modulate the functions of B and T cells. Eosinophils also communicate with a range of innate immune cells (such as mast cells, dendritic cells, macrophages and neutrophils). Eosinophils serve to bridge innate and adaptive immunity by regulating the production of chemoattractants and cytokines (including CC-chemokine ligand 17 (CCL17), CCL22, a proliferation-inducing ligand (APRIL) and IL-6) and via antigen presentation.
-
Both successful and unsuccessful attempts to target eosinophils have yielded remarkable insights into their contribution to disease pathogenesis. Many eosinophil-associated inflammatory conditions have been shown to be heterogeneous in nature. As such, successful therapeutic strategies will depend on the correlation of disease activity with dysregulated eosinophil function as well as the identification of the crucial molecules that regulate eosinophil accumulation in the affected tissues.
Abstract
This Review describes the unique biology of the eosinophil. The authors explain how eosinophils interact with other leukocyte populations to promote protective immunity following infection. They also discuss the pathological roles of eosinophils in allergic-type diseases, such as asthma and the hypereosinophilic syndromes.
Abstract
Eosinophils have been traditionally perceived as terminally differentiated cytotoxic effector cells. Recent studies have profoundly altered this simplistic view of eosinophils and their function. New insights into the molecular pathways that control the development, trafficking and degranulation of eosinophils have improved our understanding of the immunomodulatory functions of these cells and their roles in promoting homeostasis. Likewise, recent developments have generated a more sophisticated view of how eosinophils contribute to the pathogenesis of different diseases, including asthma and primary hypereosinophilic syndromes, and have also provided us with a more complete appreciation of the activities of these cells during parasitic infection.
Related collections
Most cited references150
- Record: found
- Abstract: found
- Article: not found
Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial.
- Record: found
- Abstract: found
- Article: not found
Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161.
- Record: found
- Abstract: found
- Article: not found

