- Record: found
- Abstract: found
- Article: found
Inflammasome Deletion Promotes Anti-tumor NK Cell Function in an IL-1/IL-18 Independent Way in Murine Invasive Breast Cancer
Read this article at
Abstract
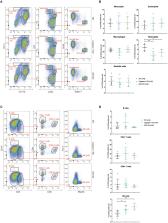
Inflammasomes are molecular complexes that trigger an inflammatory response upon detection of pathogens or danger signals. Recent studies suggest that they are also involved in cancer progression. However, their roles during tumorigenesis remain poorly understood and controversial. Here, we investigated whether inflammasome activation supports mammary tumor growth. Using mouse models of invasive breast cancer, our results demonstrate that the absence of a functional inflammasome impairs tumor growth. Importantly, tumors implanted into inflammasome-deficient mice recruited significantly less neutrophils and more natural killer (NK) cells, and these latter cells displayed a more active phenotype. Interestingly, NK cell depletion abolished the anti-tumoral effect observed in inflammasome-deficient mice, although inflammasome-regulated cytokine neutralization had no effect. Thus, our work identifies a novel role for the inflammasome in supporting mammary tumor growth by attenuating NK cell recruitment and activity. These results suggest that inflammasome inhibition could be a putative target for treating invasive breast cancers.
Related collections
Most cited references36

- Record: found
- Abstract: found
- Article: found
NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control
- Record: found
- Abstract: not found
- Article: not found
Targeting the NLRP3 inflammasome in inflammatory diseases
- Record: found
- Abstract: found
- Article: not found