- Record: found
- Abstract: found
- Article: found
Antioxidant protection of gallic acid against toxicity induced by Pb in blood, liver and kidney of rats
Read this article at
Abstract
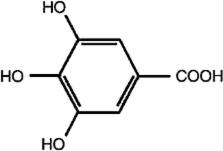
The effect of the antioxidant gallic acid (GA) on Pb toxicity in blood, liver and kidney was investigated in the present study. Rats Wistar received Pb nitrate (50 mg/Kg/day, i.p., 5 days) followed by GA (13.5 mg/Kg, p.o., 3 days) or a chelating agent (EDTA, 55 mg/Kg, i.p.). As result, Pb decreased body weight, hematocrit and blood δ-aminolevulinic acid dehydratase (ALA-D) activity. In addition, high Pb levels were observed in blood and tissues, together with increased (1) lipid peroxidation in erythrocytes, plasma and tissues, (2) protein oxidation in tissues and (3) plasma aspartate transaminase (AST) levels. These changes were accompanied by decreasing in antioxidant defenses, like superoxide dismutase (SOD) activity in tissues and catalase (CAT) activity and reduced glutathione (GSH) in liver. GA was able to reverse Pb-induced decrease in body weight and ALA-D activity, as well as Pb-induced oxidative damages and most antioxidant alterations, however it did not decrease Pb bioaccumulation herein as EDTA did. Furthermore, EDTA did not show antioxidant protection in Pb-treated animals as GA did. In conclusion, GA decreased Pb-induced oxidative damages not by decreasing Pb bioaccumulation, but by improving antioxidant defenses, thus GA may be promising in the treatment of Pb intoxications.
Related collections
Most cited references51
- Record: found
- Abstract: not found
- Article: not found
Determination of carbonyl content in oxidatively modified proteins.
- Record: found
- Abstract: found
- Article: not found
Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage.
- Record: found
- Abstract: found
- Article: not found