- Record: found
- Abstract: found
- Article: found
Changes in the expression and subcellular distribution of galectin-3 in clear cell renal cell carcinoma
Read this article at
Abstract
Background
Clear cell renal cell carcinoma, a solid growing tumor, is the most common tumor in human kidney. Evaluating the usefulness of β-galactoside binding galectin-3 as a diagnostic marker for this type of cancer could open avenues for preventive and therapeutic strategies by employing specific inhibitors of the lectin. To study a putative correlation between the extent of galectin-3 and the development of clear cell renal cell carcinoma, we monitored the quantity and distribution of this lectin in tissue samples from 39 patients.
Methods
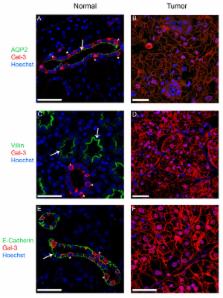
Galectin-3 concentrations in normal, intermediate and tumor tissues were examined by immunofluorescence microscopy and on immunoblots with antibodies directed against galectin-3 and renal control proteins. The cell nuclei were isolated to determine quantities of galectin-3 that were transferred into this compartment in normal or tumor samples.
Results
Immunofluorescence data revealed a mosaic pattern of galectin-3 expression in collecting ducts and distal tubules of normal kidney. Galectin-3 expression was significantly increased in 79% of tumor samples as compared to normal tissues. Furthermore, we observed an increase in nuclear translocation of the lectin in tumor tissues.
Conclusions
Our data indicate that changes in the cellular level of galectin-3 correlate with the development of clear cell renal cell carcinoma, which is in line with previously published data on this specific type of tumor. In most of these studies the lectin tends to be highly expressed in tumor tissues. Furthermore, this study suggests that the increase in the proportion of galectin-3 affects the balance from a cytosolic distribution towards translocation into the nucleus.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Galectins as modulators of tumour progression.
- Record: found
- Abstract: found
- Article: not found
Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium.
- Record: found
- Abstract: found
- Article: not found