- Record: found
- Abstract: found
- Article: found
Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-α-PD-L1 pathway
Read this article at
Abstract
Background
Mast cells are prominent components of solid tumors and exhibit distinct phenotypes in different tumor microenvironments. However, the nature, regulation, function, and clinical relevance of mast cells in human gastric cancer (GC) are presently unknown.
Methods
Flow cytometry analyses were performed to examine level and phenotype of mast cells in samples from 114 patients with GC. Multivariate analysis of prognostic factors for overall survival was performed using the Cox proportional hazards model. Kaplan-Meier plots for patient survival were performed using the log-rank test. Mast cells, T cells and tumor cells were isolated or generated, stimulated and/or cultured for in vitro and in vivo function assays.
Results
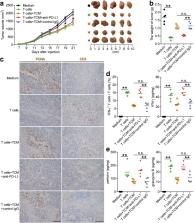
Patients with GC showed a significantly higher mast cell infiltration in tumors. Mast cell levels increased with tumor progression and independently predicted reduced overall survival. These tumor-infiltrating mast cells accumulated in tumors by CXCL12-CXCR4 chemotaxis. Intratumoral mast cells expressed higher immunosuppressive molecule programmed death-ligand 1 (PD-L1), and mast cells induced by tumors strongly express PD-L1 proteins in both time-dependent and dose-dependent manners. Significant correlations were found between the levels of PD-L1 + mast cells and pro-inflammatory cytokine TNF-α in GC tumors, and tumor-derived TNF-α activated NF-κB signaling pathway to induce mast cell expression of PD-L1. The tumor-infiltrating and tumor-conditioned mast cells effectively suppressed normal T-cell immunity through PD-L1 in vitro, and tumor-conditioned mast cells contributed to the suppression of T-cell immunity and the growth of human GC tumors in vivo; the effect could be reversed by blocking PD-L1 on these mast cells.
Related collections
Most cited references31
- Record: found
- Abstract: not found
- Article: not found
Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer

- Record: found
- Abstract: found
- Article: found
Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway
