- Record: found
- Abstract: found
- Article: found
Regulation of A disintegrin and metalloproteinase (ADAM) family sheddases ADAM10 and ADAM17: The emerging role of tetraspanins and rhomboids
Read this article at
Abstract
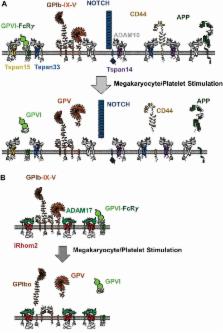
A disintegrin and metalloprotease (ADAM) 10 and ADAM17 are ubiquitous transmembrane “molecular scissors” which proteolytically cleave, or shed, the extracellular regions of other transmembrane proteins. ADAM10 is essential for development because it cleaves Notch proteins to induce Notch signaling and regulate cell fate decisions. ADAM17 is regarded as a first line of defense against injury and infection, by releasing tumor necrosis factor α (TNFα) to promote inflammation and epidermal growth factor (EGF) receptor ligands to maintain epidermal barrier function. However, the regulation of ADAM10 and ADAM17 trafficking and activation are not fully understood. This review will describe how the TspanC8 subgroup of tetraspanins (Tspan5, 10, 14, 15, 17, and 33) and the iRhom subgroup of protease-inactive rhomboids (iRhom1 and 2) have emerged as important regulators of ADAM10 and ADAM17, respectively. In particular, they are required for the enzymatic maturation and trafficking to the cell surface of the ADAMs, and there is evidence that different TspanC8s and iRhoms target the ADAMs to distinct substrates. The TspanC8s and iRhoms have not been studied functionally on platelets. On these cells, ADAM10 is the principal sheddase for the platelet collagen receptor GPVI, and the regulatory TspanC8s are Tspan14, 15, and 33, as determined from proteomic data. Platelet ADAM17 is the sheddase for the von Willebrand factor (vWF) receptor GPIb, and iRhom2 is the only iRhom that is expressed. Induced shedding of either GPVI or GPIb has therapeutic potential, since inhibition of either receptor is regarded as a promising anti-thrombotic therapy. Targeting of Tspan14, 15, or 33 to activate platelet ADAM10, or iRhom2 to activate ADAM17, may enable such an approach to be realized, without the toxic side effects of activating the ADAMs on every cell in the body.
Related collections
Most cited references72
- Record: found
- Abstract: found
- Article: not found
The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways.
- Record: found
- Abstract: found
- Article: not found
ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation.
- Record: found
- Abstract: found
- Article: not found