- Record: found
- Abstract: found
- Article: found
Regional differences in Alzheimer’s disease pathology confound behavioural rescue after amyloid-β attenuation
Read this article at
Abstract
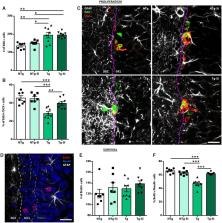
Aβ clearance does not improve cognition in patients with Alzheimer’s disease. Using a transgenic rat model, Morrone et al. show that Aβ attenuation has no effect on entorhinal tau or entorhinal-hippocampal network dysfunction, explaining the persistence of spatial memory deficits. However, it improves hippocampal function, thereby rescuing pattern separation and executive function.
Abstract
Failure of Alzheimer’s disease clinical trials to improve or stabilize cognition has led to the need for a better understanding of the driving forces behind cognitive decline in the presence of active disease processes. To dissect contributions of individual pathologies to cognitive function, we used the TgF344-AD rat model, which recapitulates the salient hallmarks of Alzheimer’s disease pathology observed in patient populations (amyloid, tau inclusions, frank neuronal loss, and cognitive deficits). scyllo-Inositol treatment attenuated amyloid-β peptide in disease-bearing TgF344-AD rats, which rescued pattern separation in the novel object recognition task and executive function in the reversal learning phase of the Barnes maze. Interestingly, neither activities of daily living in the burrowing task nor spatial memory in the Barnes maze were rescued by attenuating amyloid-β peptide. To understand the pathological correlates leading to behavioural rescue, we examined the neuropathology and in vivo electrophysiological signature of the hippocampus. Amyloid-β peptide attenuation reduced hippocampal tau pathology and rescued adult hippocampal neurogenesis and neuronal function, via improvements in cross-frequency coupling between theta and gamma bands. To investigate mechanisms underlying the persistence of spatial memory deficits, we next examined neuropathology in the entorhinal cortex, a region whose input to the hippocampus is required for spatial memory. Reduction of amyloid-β peptide in the entorhinal cortex had no effect on entorhinal tau pathology or entorhinal-hippocampal neuronal network dysfunction, as measured by an impairment in hippocampal response to entorhinal stimulation. Thus, rescue or not of cognitive function is dependent on regional differences of amyloid-β, tau and neuronal network dysfunction, demonstrating the importance of staging disease in patients prior to enrolment in clinical trials. These results further emphasize the need for combination therapeutic approaches across disease progression.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Microstructure of a spatial map in the entorhinal cortex.
- Record: found
- Abstract: found
- Article: not found
Adult hippocampal neurogenesis and cognitive flexibility — linking memory and mood
- Record: found
- Abstract: found
- Article: not found
