- Record: found
- Abstract: found
- Article: found
Facile and Rapid Room-Temperature Electrosynthesis and Controlled Surface Growth of Fe-MIL-101 and Fe-MIL-101-NH 2

Read this article at
Abstract
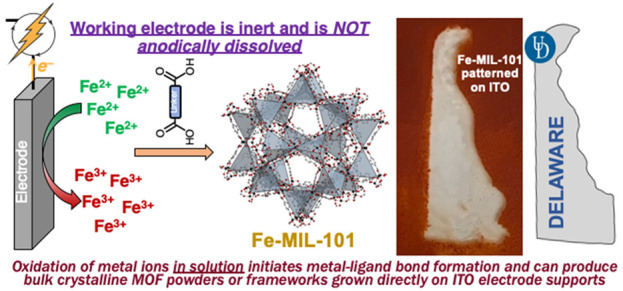
The electrochemical synthesis of metal–organic frameworks (MOFs) has been widely explored but has involved indirect routes, including anodic dissolution of solid metal electrodes or the use of interfacial redox chemistry to generate base equivalents and drive MOF assembly. These methods are limited in scope, as the former relies on the use of an anode consisting of the metal ion to be incorporated into the MOF, and the latter relies on the compatibility of the metal/ligand solution with the probase that is subsequently oxidized or reduced. We report the facile, direct electrochemical syntheses of four iron-based MOFs via controlled potential oxidation of dissolved metal cations. Oxidation of Fe(II) at +0.75 V (vs Ag/Ag +) in a solution containing 2,6-lutidine and terephthalic acid affords highly crystalline Fe-MIL-101. Controlled potential electrolysis with carboxy-functionalized ITO affords Fe-MIL-101 grown directly on the surface of modified electrodes. The methods we report herein represent the first general routes that employ interfacial electrochemistry to alter the oxidation state of metal ions dissolved in solution to directly trigger MOF formation. The reported method is functional group tolerant and will be broadly applicable to the bulk synthesis or surface growth of a range of MOFs based on metal ions with accessible oxidation states.
Abstract
The first direct electrosynthesis of metal−organic frameworks from soluble metal salts is reported and is leveraged to prepare high-quality MOF samples and MOF thin films on inert electrode supports.
Related collections
Most cited references44
- Record: found
- Abstract: not found
- Article: not found
Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites.
- Record: found
- Abstract: not found
- Article: not found
Design and synthesis of an exceptionally stable and highly porous metal-organic framework
- Record: found
- Abstract: found
- Article: not found