- Record: found
- Abstract: found
- Article: found
Gene Therapy for Parkinson’s Disease, An Update
Read this article at
Abstract
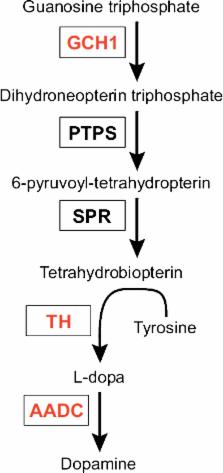
The current mainstay treatment of Parkinson’s disease (PD) consists of dopamine replacement therapy which, in addition to causing several side effects, does not delay disease progression. The field of gene therapy offers a potential means to improve current therapy. The present review gives an update of the present status of gene therapy for PD. Both non-disease and disease modifying transgenes have been tested for PD gene therapy in animal and human studies. Non-disease modifying treatments targeting dopamine or GABA synthesis have been successful and promising at improving PD symptomatology in randomized clinical studies, but substantial testing remains before these can be implemented in the standard clinical treatment repertoire. As for disease modifying targets that theoretically offer the possibility of slowing the progression of disease, several neurotrophic factors show encouraging results in preclinical models (e.g., neurturin, GDNF, BDNF, CDNF, VEGF-A). However, so far, clinical trials have only tested neurturin, and, unfortunately, no trial has been able to meet its primary endpoint. Future clinical trials with neurotrophic factors clearly deserve to be conducted, considering the still enticing goal of actually slowing the disease process of PD. As alternative types of gene therapy, opto- and chemogenetics might also find future use in PD treatment and novel genome-editing technology could also potentially be applied as individualized gene therapy for genetic types of PD.
Related collections
Most cited references128
- Record: found
- Abstract: found
- Article: not found
TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity.
- Record: found
- Abstract: found
- Article: not found