- Record: found
- Abstract: found
- Article: found
Synthesis and Characterization of Novel Methyl (3)5-( N-Boc-piperidinyl)-1 H-pyrazole-4-carboxylates

Read this article at
Abstract
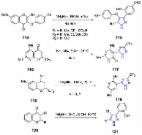
Series of methyl 3- and 5-( N-Boc-piperidinyl)-1 H-pyrazole-4-carboxylates were developed and regioselectively synthesized as novel heterocyclic amino acids in their N-Boc protected ester form for achiral and chiral building blocks. In the first stage of the synthesis, piperidine-4-carboxylic and ( R)- and ( S)-piperidine-3-carboxylic acids were converted to the corresponding β-keto esters, which were then treated with N, N-dimethylformamide dimethyl acetal. The subsequent reaction of β-enamine diketones with various N-mono-substituted hydrazines afforded the target 5-( N-Boc-piperidinyl)-1 H-pyrazole-4-carboxylates as major products, and tautomeric NH-pyrazoles prepared from hydrazine hydrate were further N-alkylated with alkyl halides to give 3-( N-Boc-piperidinyl)-1 H-pyrazole-4-carboxylates. The structures of the novel heterocyclic compounds were confirmed by 1H-, 13C-, and 15N-NMR spectroscopy and HRMS investigation.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules.
- Record: found
- Abstract: found
- Article: found
Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review
- Record: found
- Abstract: not found
- Article: not found