- Record: found
- Abstract: found
- Article: found
Norepinephrine stimulates glycogenolysis in astrocytes to fuel neurons with lactate
Read this article at
Abstract
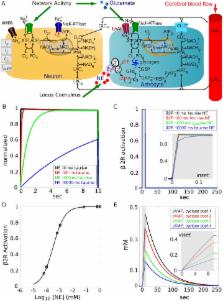
The mechanism of rapid energy supply to the brain, especially to accommodate the heightened metabolic activity of excited states, is not well-understood. We explored the role of glycogen as a fuel source for neuromodulation using the noradrenergic stimulation of glia in a computational model of the neural-glial-vasculature ensemble (NGV). The detection of norepinephrine (NE) by the astrocyte and the coupled cAMP signal are rapid and largely insensitive to the distance of the locus coeruleus projection release sites from the glia, implying a diminished impact for volume transmission in high affinity receptor transduction systems. Glucosyl-conjugated units liberated from glial glycogen by NE-elicited cAMP second messenger transduction winds sequentially through the glycolytic cascade, generating robust increases in NADH and ATP before pyruvate is finally transformed into lactate. This astrocytic lactate is rapidly exported by monocarboxylate transporters to the associated neuron, demonstrating that the astrocyte-to-neuron lactate shuttle activated by glycogenolysis is a likely fuel source for neuromodulation and enhanced neural activity. Altogether, the energy supply for both astrocytes and neurons can be supplied rapidly by glycogenolysis upon neuromodulatory stimulus.
Author summary
Although efficient compared to computers, the human brain utilizes energy at 10-fold the rate of other organs by mass. How the brain is supplied with sufficient on-demand energy to support its activity in the absence of neuronal storage capacity remains unknown. Neurons are not capable of meeting their own energy requirements, instead energy supply in the brain is managed by an oligocellular cartel composed of neurons, glia and the local vasculature (NGV), wherein glia can provide the ergogenic metabolite lactate to the neuron in a process called the astrocyte-to-neuron shuttle (ANLS). The only means of energy storage in the brain is glycogen, a polymerized form of glucose that is localized largely to astrocytes, but its exact role and conditions of use are not clear. In this computational model we show that neuromodulatory stimulation by norepinephrine induces astrocytes to recover glucosyl subunits from glycogen for use in a glycolytic process that favors the production of lactate. The ATP and NADH produced support metabolism in the astrocyte while the lactate is exported to feed the neuron. Thus, rapid energy demands by both neurons and glia in a stimulated brain can be met by glycogen mobilization.
Related collections
Most cited references129
- Record: found
- Abstract: found
- Article: not found
Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation.
- Record: found
- Abstract: found
- Article: not found
Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization.
- Record: found
- Abstract: found
- Article: not found