- Record: found
- Abstract: found
- Article: found
Alteration of Vascular Responsiveness to Uridine Adenosine Tetraphosphate in Aortas Isolated from Male Diabetic Otsuka Long-Evans Tokushima Fatty Rats: The Involvement of Prostanoids
Read this article at
Abstract
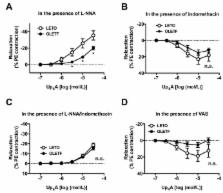
We investigated whether responsiveness to dinucleotide uridine adenosine tetraphosphate (Up 4A) was altered in aortas from type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats compared with those from age-matched control Long-Evans Tokushima Otsuka (LETO) rats at the chronic stage of disease. In OLETF aortas, we observed the following: (1) Up 4A-induced contractions were lower than those in the LETO aortas under basal conditions, (2) slight relaxation occurred due to Up 4A, but this was not observed in phenylephrine-precontracted LETO aortas, (3) acetylcholine-induced relaxation was reduced (vs. LETO), and (4) prostanoid release (prostaglandin (PG)F 2α, thromboxane (Tx)A 2 metabolite, and PGE 2) due to Up 4A was decreased (vs. LETO). Endothelial denudation suppressed Up 4A-induced contractions in the LETO group, but increased the contractions in the OLETF group. Under nitric oxide synthase (NOS) inhibition, Up 4A induced contractions in phenylephrine-precontracted aortas; this effect was greater in the LETO group (vs. the OLETF group). The relaxation response induced by Up 4A was unmasked by cyclooxygenase inhibitors, especially in the LETO group, but this effect was abolished by NOS inhibition. These results suggest that the relaxant component of the Up 4A-mediated response was masked by prostanoids in the LETO aortas and that the LETO and OLETF rats presented different contributions of the endothelium to the response.
Related collections
Most cited references64
- Record: found
- Abstract: found
- Article: not found
Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain.
- Record: found
- Abstract: found
- Article: not found
Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator.
- Record: found
- Abstract: found
- Article: not found