- Record: found
- Abstract: found
- Article: not found
Biomimetic proteolipid vesicles for targeting inflamed tissues
Abstract
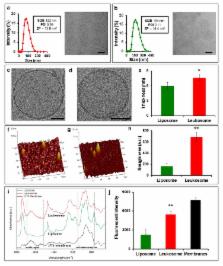
A multitude of micro- and nanoparticles have been developed to improve the delivery of systemically administered pharmaceuticals, which are subject to a number of biological barriers that limit their optimal biodistribution. Bioinspired drug-delivery carriers formulated by bottom-up or top-down strategies have emerged as an alternative approach to evade the mononuclear phagocytic system and facilitate the transport across the endothelial vessel wall. Here, we describe a method that leverages the advantages of bottom-up and top-down strategies to incorporate proteins derived from the leukocyte plasma membrane into lipid nanoparticles. The resulting proteolipid vesicles - which we refer to as leukosomes - retained the versatility and physicochemical properties typical of liposomal formulations, preferentially targeted inflamed vasculature, enabled the selective and effective delivery of dexamethasone to inflamed tissues, and reduced phlogosis in a localized model of inflammation.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies.
- Record: found
- Abstract: found
- Article: not found
Bio-inspired, bioengineered and biomimetic drug delivery carriers.
- Record: found
- Abstract: found
- Article: not found
