- Record: found
- Abstract: found
- Article: found
CXCL14 Preferentially Synergizes With Homeostatic Chemokine Receptor Systems
Read this article at
Abstract
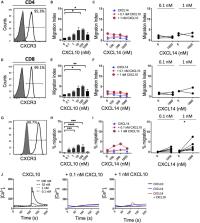
Reflecting their importance in immunity, the activity of chemokines is regulated on several levels, including tissue and context-specific expression and availability of their cognate receptor on target cells. Chemokine synergism, affecting both chemokine and chemokine receptor function, has emerged as an additional control mechanism. We previously demonstrated that CXCL14 is a positive allosteric modulator of CXCR4 in its ability to synergize with CXCL12 in diverse cellular responses. Here, we have extended our study to additional homeostatic, as well as a selection of inflammatory chemokine systems. We report that CXCL14 strongly synergizes with low (sub-active) concentrations of CXCL13 and CCL19/CCL21 in in vitro chemotaxis with immune cells expressing the corresponding receptors CXCR5 and CCR7, respectively. CXCL14 by itself was inactive, not only on cells expressing CXCR5 or CCR7 but also on cells expressing any other known conventional or atypical chemokine receptor, as assessed by chemotaxis and/or β-arrestin recruitment assays. Furthermore, synergistic migration responses between CXCL14 and inflammatory chemokines CXCL10/CXCL11 and CCL5, targeting CXCR3 and CCR5, respectively, were marginal and occasional synergistic Ca 2+ flux responses were observed. CXCL14 bound to 300-19 cells and interfered with CCL19 binding to CCR7-expressing cells, suggesting that these cellular interactions contributed to the reported CXCL14-mediated synergistic activities. We propose a model whereby tissue-expressed CXCL14 contributes to cell localization under steady-state conditions at sites with prominent expression of homeostatic chemokines.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
Chemokines and chemokine receptors: positioning cells for host defense and immunity.
- Record: found
- Abstract: found
- Article: not found
A new generation of Ca2+ indicators with greatly improved fluorescence properties.
- Record: found
- Abstract: found
- Article: not found