- Record: found
- Abstract: found
- Article: found
Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis
letter
14 May 2020
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
To the Editor:
Coronavirus disease 2019 (COVID‐19) is widely spread and poses a critical threat to
global health.
1
Prominent changes in coagulation function in severe patients of COVID‐19 have been
reported in a recent study.
2
Therefore, we conducted this quantitative meta‐analysis to explore the difference
in blood coagulation parameters between severe and mild cases of COVID‐19.
Literature published from December 1, 2019 to March 30, 2020 was searched systematically
using PubMed and Embase without language limits. The keywords were: coronavirus, laboratory,
clinical manifestations, clinical characteristics, and clinical features. All documents
comparing information on coagulation parameters between mild and severe cases of COVID‐19
patients were finally referred to in our meta‐analysis. The pooled standardised mean
difference (SMD) and 95% confidence interval (CI) were computed by applying the random‐effect
model using Stata software (STATA 14.0, Stata Corp, College Station, TX, USA). The
study quality was measured by adopting an 11‐item checklist, which was suggested by
the Agency for Healthcare Research and Quality (AHRQ).
Table I displays the main characteristics of the included studies. Nine studies, including
one study from medRxiv, with 1105 patients were eventually included for detailed evaluation.
Platelet count (PLT), activated partial thromboplastin time (APTT), prothrombin time
(PT) and D‐dimer (D‐D) levels were available in five, six, six and eight studies,
respectively. All the studies were conducted in China. Quality score varied from 3
to 7 points, with a mean of 5·4 (Table I). All the studies were of moderate quality,
except one of low quality.
Table 1
Study characteristics.
Study
N (male %)
n
Age (years)
Severity criteria
QS
Coagulation parameters*
PLT (109/l)
APTT (s)
PT (s)
D‐D (mg/l)
Han et al.
2
94 (51·0)
45
NA
Trail version 5
5
NA
29·5 (3·2)/28·6 (2·7)
12·7 (1·1)/12·2 (0·9)
19·3 (34·5)/2·1 ( 2·9)
Huang et al.
4
41 (73·0)
13
49·0 (12·6)
ICU and non‐ICU
6
196·0 (72·6)/149·0 (97·8)
26·2 (8·4)/27·7 (6·9)
12·2 (1·6)/10·7 (1·7)
2·4 (10·2)/0·5 (0·4)
Liu et al.
5
30 (33·3)
4
35·0 (8·0)
Trail version 5
5
NA
NA
NA
1·5 (1·2)/0·3 (0·1)
Mao et al.
6
214 (40·7)
88
52·7 (15·5)
WHO interim guideline
5
204·5 (413·3)/219·0 (400·7)
NA
NA
0·9 (14·7)/0·4 (6·3)
Peng et al.
7
112 (47·3)
16
62·0 (8·9)
Standard version
6
NA
36·5 (8·4)/35·8 (6·2)
13·9 (1·6)/13·0 (1·2)
NA
Wan et al.
8
135 (53·3)
40
47·0 (14·1)
Trail version 5
6
147·0 (70·4)/170·0 (72·6)
29·7 (9·8)/26·6 (3·2)
11·3 (0·8)/10·8 (0·7)
0·6 (0·5)/0·3 (0·2)
Wang et al.
9
138 (54·3)
36
56·0 (19·2)
ICU and non‐ICU
7
142·0 (61·5)/165·0 (46·7)
30·4 (4·1)/31·7 (2·9)
13·2 (1·6)/12·9 (0·8)
0·4 (0·8)/0·2 (0·1)
Wu et al.
10
201 (63·7)
84
51·0 (12·3)
With and without ARDS
6
187·0 (94·1)/178·0 (73·7)
26·0 (9·2)/29·5 (5·4)
11·7 (1·0)/10·6 (1·1)
1·2 (3·6)/0·5 (0·4)
Zhang et al.
1
140 (50·7)
58
57·0 (45·9)
Trail version 5
3
NA
NA
NA
0·4 (1·6)/0·2 (0·1)
N, number of included patients; n, number of severe patients; QS, quality score; NA,
not available; PLT, platelet, reference interval 125–350 × 109/l; APTT, activated
partial thromboplastin time, reference interval 25·1–36·5 s; PT, prothrombin time,
reference interval 9·4–12·5 s; D‐D, D‐dimer, reference interval 0–0·5 mg/l; ICU, intensive
care unit; ARDS, acute respiratory distress syndrome.
*
Data presented as severe/mild COVID‐19 patients; data as given as mean (standard derivation).
John Wiley & Sons, Ltd
This article is being made freely available through PubMed Central as part of the
COVID-19 public health emergency response. It can be used for unrestricted research
re-use and analysis in any form or by any means with acknowledgement of the original
source, for the duration of the public health emergency.
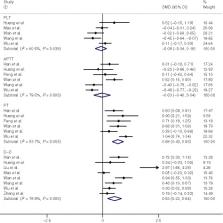
The main difference in coagulation function between severe and mild COVID‐19 patients
is shown in Fig 1. Pooled results revealed that PT and D‐D levels were significantly
higher in patients with severe COVID‐19 (0·68, 95% CI = 0·43–0·93, I
2 = 53·7%; 0·53, 95% CI = 0·22–0·84, I
2 = 78·9%, respectively). However, no significant difference in PLT and APTT values
between severe and mild patients was observed (−0·08, 95% CI = −0·34 to 0·18, I
2 = 60·5%; −0·03, 95% CI = −0·40 to 0·34, I
2 = 79·5%, respectively). Increasing values of D‐D and PT support the notion that
disseminated intravascular coagulation (DIC) may be common in COVID‐19 patients.
2
In addition, the rise of D‐D level also indicates secondary fibrinolysis conditions
in these patients. According to Berri et al.,
3
fibrin clot formation helps people to fight against influenza virus infections. Hence,
fibrinolysis may potentially induce following severe COVID‐19 infection. Future studies
should aim to discover more biomarkers of severe cases of COVID‐19, and studies exploring
the underlying mechanism of deranged coagulation function in COVID‐19 are urgently
needed. The haemostatic system might be explored for underlying treatment against
coronavirus.
Fig 1
Forest plot of PLT, APTT, PT and D‐D levels in severe COVID‐19 patients versus mild
COVID‐19 patients.
Due to the lack of sufficient study data, we cannot perform a more thorough analysis
to prove beneficial screening parameters for PLT, APTT, PT and D‐D for prediction
of severity of COVID‐19. However, we suggest that clinical practitioners pay attention
to the changes in coagulation function in COVID‐19 patients on a daily basis.
Related collections
Most cited references10
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
Chaolin Huang, Yeming Wang, Xingwang Li … (2020)
- Record: found
- Abstract: found
- Article: found
Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China
Dawei Wang, Bo Hu, Fangfang Zhu … (2020)
- Record: found
- Abstract: found
- Article: not found
Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China
Chaomin Wu, Xiaoyan Chen, Yanping Cai … (2020)
