- Record: found
- Abstract: found
- Article: found
Facioscapulohumeral muscular dystrophy: genetics, gene activation and downstream signalling with regard to recent therapeutic approaches: an update
Read this article at
Abstract
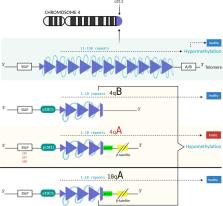
Whilst a disease-modifying treatment for Facioscapulohumeral muscular dystrophy (FSHD) does not exist currently, recent advances in complex molecular pathophysiology studies of FSHD have led to possible therapeutic approaches for its targeted treatment. Although the underlying genetics of FSHD have been researched extensively, there remains an incomplete understanding of the pathophysiology of FSHD in relation to the molecules leading to DUX4 gene activation and the downstream gene targets of DUX4 that cause its toxic effects. In the context of the local proximity of chromosome 4q to the nuclear envelope, a contraction of the D4Z4 macrosatellite induces lower methylation levels, enabling the ectopic expression of DUX4. This disrupts numerous signalling pathways that mostly result in cell death, detrimentally affecting skeletal muscle in affected individuals. In this regard different options are currently explored either to suppress the transcription of DUX4 gene, inhibiting DUX4 protein from its toxic effects, or to alleviate the symptoms triggered by its numerous targets.
Related collections
Most cited references226
- Record: found
- Abstract: found
- Article: not found