- Record: found
- Abstract: found
- Article: found
The Role of the Pathogen Dose and PI3Kγ in Immunometabolic Reprogramming of Microglia for Innate Immune Memory
Read this article at
Abstract
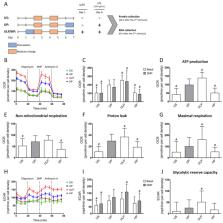
Microglia, the innate immune cells of the CNS, exhibit long-term response changes indicative of innate immune memory (IIM). Our previous studies revealed IIM patterns of microglia with opposing immune phenotypes: trained immunity after a low dose and immune tolerance after a high dose challenge with pathogen-associated molecular patterns (PAMP). Compelling evidence shows that innate immune cells adopt features of IIM via immunometabolic control. However, immunometabolic reprogramming involved in the regulation of IIM in microglia has not been fully addressed. Here, we evaluated the impact of dose-dependent microglial priming with ultra-low (ULP, 1 fg/mL) and high (HP, 100 ng/mL) lipopolysaccharide (LPS) doses on immunometabolic rewiring. Furthermore, we addressed the role of PI3Kγ on immunometabolic control using naïve primary microglia derived from newborn wild-type mice, PI3Kγ-deficient mice and mice carrying a targeted mutation causing loss of lipid kinase activity. We found that ULP-induced IIM triggered an enhancement of oxygen consumption and ATP production. In contrast, HP was followed by suppressed oxygen consumption and glycolytic activity indicative of immune tolerance. PI3Kγ inhibited glycolysis due to modulation of cAMP-dependent pathways. However, no impact of specific PI3Kγ signaling on immunometabolic rewiring due to dose-dependent LPS priming was detected. In conclusion, immunometabolic reprogramming of microglia is involved in IIM in a dose-dependent manner via the glycolytic pathway, oxygen consumption and ATP production: ULP (ultra-low-dose priming) increases it, while HP reduces it.
Related collections
Most cited references64
- Record: found
- Abstract: found
- Article: not found
Analyzing real-time PCR data by the comparative C(T) method.
- Record: found
- Abstract: found
- Article: not found
Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo.
- Record: found
- Abstract: found
- Article: not found