- Record: found
- Abstract: found
- Article: found
The Impact of Inflammation on Metabolomic Profiles in Patients With Arthritis
Read this article at
Abstract
Objective. Inflammatory arthritis is associated with systemic manifestations including alterations in metabolism. We used nuclear magnetic resonance (NMR) spectroscopy–based metabolomics to assess metabolic fingerprints in serum from patients with established rheumatoid arthritis (RA) and those with early arthritis.
Methods. Serum samples were collected from newly presenting patients with established RA who were naive for disease-modifying antirheumatic drugs, matched healthy controls, and 2 groups of patients with synovitis of ≤3 months' duration whose outcomes were determined at clinical followup. Serum metabolomic profiles were assessed using 1-dimensional 1H-NMR spectroscopy. Discriminating metabolites were identified, and the relationships between metabolomic profiles and clinical variables including outcomes were examined.
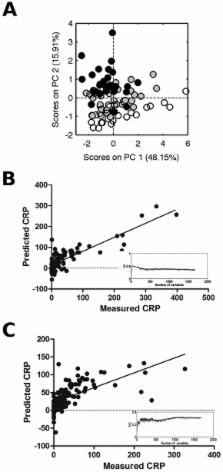
Results. The serum metabolic fingerprint in established RA was clearly distinct from that of healthy controls. In early arthritis, we were able to stratify the patients according to the level of current inflammation, with C-reactive protein correlating with metabolic differences in 2 separate groups ( P < 0.001). Lactate and lipids were important discriminators of inflammatory burden in both early arthritis patient groups. The sensitivities and specificities of models to predict the development of either RA or persistent arthritis in patients with early arthritis were low.
Conclusion. The metabolic fingerprint reflects inflammatory disease activity in patients with synovitis, demonstrating that underlying inflammatory processes drive significant changes in metabolism that can be measured in the peripheral blood. The identification of metabolic alterations may provide insights into disease mechanisms operating in patients with inflammatory arthritis.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis.
- Record: found
- Abstract: found
- Article: not found
Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression.
- Record: found
- Abstract: found
- Article: not found