- Record: found
- Abstract: found
- Article: found
Tanshinone IIA protects mice against atherosclerotic injury by activating the TGF-β/PI3K/Akt/eNOS pathway
Read this article at
Objective
Explored the mechanism of action of tanshinone IIA (TIIA) against atherosclerosis.
Methods
ApoE −/− mice were divided into two groups of 10: model and TIIA. A control group of 10 wild-type mice was created. ApoE −/− mice were fed a high-fat diet for 12 weeks. The TIIA group received TIIA once daily. Mice were anesthetized, blood collected by cardiac puncture, and the aortic sinus/arch collected for histology and molecular studies, respectively.
Results
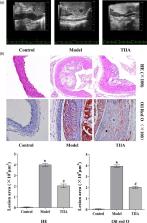
Mice intima in the model group had large areas of plaque formation, serum levels of total cholesterol (TC), triglycerides, and low-density lipoprotein-cholesterol (LDL-C) increased significantly, and high-density lipoprotein-cholesterol (HDL-C) levels decreased significantly in the model group after 12 weeks. Staining [hematoxylin and eosin (H&E), Oil-Red-O] showed that the aorta had lesions, a higher degree of plaque formation, and considerable lipid deposition in model-group mice. After TIIA treatment, expression of HDL-C was increased significantly and that of TC, triglycerides and LDL-C decreased significantly, and plaque size and lipid deposition improved obviously. Analyses of protein phosphorylation in aortic tissue suggested that the transforming growth factor (TGF)-β/phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/endothelial nitric oxide synthase (eNOS) pathway was activated in TIIA-treated mice.
Related collections
Most cited references27

- Record: found
- Abstract: found
- Article: found
The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling
- Record: found
- Abstract: found
- Article: not found
Hypoxia inducible factor as a therapeutic target for atherosclerosis.
- Record: found
- Abstract: found
- Article: not found