- Record: found
- Abstract: found
- Article: found
Modern Tools for Rapid Diagnostics of Antimicrobial Resistance
Read this article at
Abstract
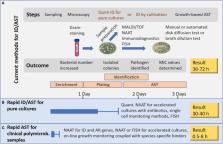
Fast, robust, and affordable antimicrobial susceptibility testing (AST) is required, as roughly 50% of antibiotic treatments are started with wrong antibiotics and without a proper diagnosis of the pathogen. Validated growth-based AST according to EUCAST or CLSI (European Committee on Antimicrobial Susceptibility Testing, Clinical Laboratory Standards Institute) recommendations is currently suggested to guide the antimicrobial therapy. Any new AST should be validated against these standard methods. Many rapid diagnostic techniques can already provide pathogen identification. Some of them can additionally detect the presence of resistance genes or resistance proteins, but usually isolated pure cultures are needed for AST. We discuss the value of the technologies applying nucleic acid amplification, whole genome sequencing, and hybridization as well as immunodiagnostic and mass spectrometry-based methods and biosensor-based AST. Additionally, we evaluate the potential of integrated systems applying microfluidics to integrate cultivation, lysis, purification, and signal reading steps. We discuss technologies and commercial products with potential for Point-of-Care Testing (POCT) and their capability to analyze polymicrobial samples without pre-purification steps. The purpose of this critical review is to present the needs and drivers for AST development, to show the benefits and limitations of AST methods, to introduce promising new POCT-compatible technologies, and to discuss AST technologies that are likely to thrive in the future.
Related collections
Most cited references129
- Record: found
- Abstract: found
- Article: not found
Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose.
- Record: found
- Abstract: found
- Article: not found
Nucleic acid sequence-based amplification.
- Record: found
- Abstract: found
- Article: not found
