- Record: found
- Abstract: found
- Article: found
Skin Autofluorescence, as a Measure of AGE Accumulation in Individuals Suffering from Chronic Plaque Psoriasis
Read this article at
Abstract
Background
Psoriasis is currently regarded as a chronic systemic inflammatory disease associated with increased cardiovascular risk. Advanced glycation end products (AGEs) contribute to the development of atherosclerosis.
Objectives
The aim of the study was the assessment of skin autofluorescence (SAF), as a measure of AGE accumulation, in individuals suffering from chronic plaque psoriasis without any comorbid conditions.
Methods
A study group consisted of 70 patients with chronic plaque psoriasis without any comorbid conditions and 59 healthy controls, matched by age and gender. AGE accumulation was assessed by SAF (AGE Reader, DiagnOptics BV) which is a validated and noninvasive technique. Relations between SAF and some clinical and laboratory data were assessed.
Results
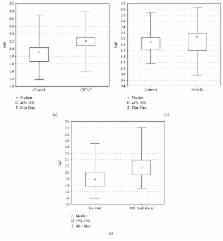
SAF was positively correlated with age both in patients with psoriasis and controls ( R = 0.722, p < 0.00001 and R = 0.613, p < 0.00001, respectively). There was significantly increased SAF in patients with psoriasis with elevated levels of C-reactive protein (CRP) and increased erythrocyte sedimentation rate (ESR) compared to controls ( p < 0.00001; p < 0.00001, respectively, after adjustment to age). Increased SAF was found in psoriatic patients with prediabetes (HbA1c 5.7–6.4%) compared to controls ( p < 0.0012, after adjustment to age).
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions.
- Record: found
- Abstract: found
- Article: not found
The 'psoriatic march': a concept of how severe psoriasis may drive cardiovascular comorbidity.
- Record: found
- Abstract: found
- Article: not found