- Record: found
- Abstract: found
- Article: found
Identification of Key Residues for Urate Specific Transport in Human Glucose Transporter 9 (hSLC2A9)
Read this article at
Abstract
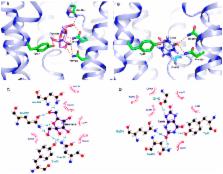
Human glucose transporter 9 (hSLC2A9) is critical in human urate homeostasis, for which very small deviations can lead to chronic or acute metabolic disorders. Human SLC2A9 is unique in that it transports hexoses as well as the organic anion, urate. This ability is in contrast to other homologous sugar transporters such as glucose transporters 1 and 5 (SLC2A1 & SLC2A5) and the xylose transporter (XylE), despite the fact that these transporters have similar protein structures. Our in silico substrate docking study has revealed that urate and fructose bind within the same binding pocket in hSLC2A9, yet with distinct orientations, and allowed us to identify novel residues for urate binding. Our functional studies confirmed that N429 is a key residue for both urate binding and transport. We have shown that cysteine residues, C181, C301 and C459 in hSLC2A9 are also essential elements for mediating urate transport. Additional data from chimæric protein analysis illustrated that transmembrane helix 7 of hSLC2A9 is necessary for urate transport but not sufficient to allow urate transport to be induced in glucose transporter 5 (hSLC2A5). These data indicate that urate transport in hSLC2A9 involves several structural elements rather than just a unique substrate binding pocket.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Crystal structure of the human glucose transporter GLUT1.
- Record: found
- Abstract: found
- Article: not found
SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout.
- Record: found
- Abstract: found
- Article: not found