- Record: found
- Abstract: found
- Article: found
Associations of circulating saturated long-chain fatty acids with risk of mild cognitive impairment and Alzheimer’s disease in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort
Read this article at
Summary
Background
No study has examined the associations between peripheral saturated long-chain fatty acids (LCFAs) and conversion from mild cognitive impairment (MCI) to Alzheimer’s disease (AD). This study aimed to examine whether circulating saturated LCFAs are associated with both risks of incident MCI from cognitively normal (CN) participants and incident AD progressed from MCI in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort.
Methods
We conducted analysis of data from older adults aged 55–90 years who were recruited at 63 sites across the USA and Canada. We examined associations between circulating saturated LCFAs (i.e., C14:0, C16:0, C18:0, C20:0) and risk for incident MCI in CN participants, and incident AD progressed from MCI.
Findings
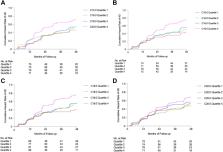
829 participants who were enrolled in ADNI-1 had data on plasma saturated LCFAs, of which 618 AD-free participants were included in our analysis (226 with normal cognition and 392 with MCI; 60.2% were men). Cox proportional-hazards models were used to account for time-to-event/censor with a 48-month follow-up period for the primary analysis. Other than C20:0, saturated LCFAs were associated with an increased risk for AD among participants with MCI at baseline (Hazard ratios (HRs) = 1.3 to 2.2, P = 0.0005 to 0.003 in fully-adjusted models). No association of C20:0 with risk of AD among participants with MCI was observed. No associations were observed between saturated LCFAs and risk for MCI among participants with normal cognition.
Related collections
Most cited references37

- Record: found
- Abstract: found
- Article: found
The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication
- Record: found
- Abstract: found
- Article: not found
Robust causal inference using directed acyclic graphs: the R package ‘dagitty’
- Record: found
- Abstract: found
- Article: not found