- Record: found
- Abstract: found
- Article: found
Cyclic di-GMP signalling and the regulation of bacterial virulence
Read this article at
Abstract
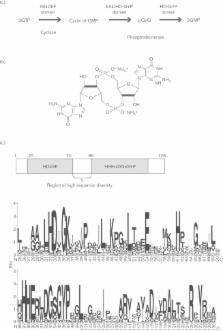
Signal transduction pathways involving the second messenger cyclic di-GMP [bis-(3′-5′)-cyclic di-guanosine monophosphate] occur widely in bacteria where they act to link perception of environmental or intracellular cues and signals to specific alterations in cellular function. Such alterations can contribute to bacterial lifestyle transitions including biofilm formation and virulence. The cellular levels of the nucleotide are controlled through the opposing activities of diguanylate cyclases (DGCs) and phosphodiesterases (PDEs). The GGDEF domain of DGCs catalyses the synthesis of cyclic di-GMP from GTP, whereas EAL or HD-GYP domains in different classes of PDE catalyse cyclic di-GMP degradation to pGpG and GMP. We are now beginning to understand how alterations in cyclic di-GMP exert a regulatory action through binding to diverse receptors or effectors that include a small ‘adaptor’ protein domain called PilZ, transcription factors and riboswitches. The regulatory action of enzymically active cyclic di-GMP signalling proteins is, however, not restricted to an influence on the level of nucleotide. Here, I will discuss our recent findings that highlight the role that protein–protein interactions involving these signalling proteins have in regulating functions that contribute to bacterial virulence.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain.
- Record: found
- Abstract: found
- Article: not found
Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid.
- Record: found
- Abstract: found
- Article: not found