- Record: found
- Abstract: found
- Article: found
Monitoring the maturation of the sarcomere network: a super-resolution microscopy-based approach
Read this article at
Abstract
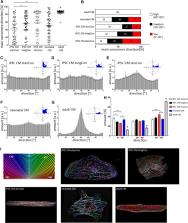
The in vitro generation of human cardiomyocytes derived from induced pluripotent stem cells (iPSC) is of great importance for cardiac disease modeling, drug-testing applications and for regenerative medicine. Despite the development of various cultivation strategies, a sufficiently high degree of maturation is still a decisive limiting factor for the successful application of these cardiac cells. The maturation process includes, among others, the proper formation of sarcomere structures, mediating the contraction of cardiomyocytes. To precisely monitor the maturation of the contractile machinery, we have established an imaging-based strategy that allows quantitative evaluation of important parameters, defining the quality of the sarcomere network. iPSC-derived cardiomyocytes were subjected to different culture conditions to improve sarcomere formation, including prolonged cultivation time and micro patterned surfaces. Fluorescent images of α-actinin were acquired using super-resolution microscopy. Subsequently, we determined cell morphology, sarcomere density, filament alignment, z-Disc thickness and sarcomere length of iPSC-derived cardiomyocytes. Cells from adult and neonatal heart tissue served as control. Our image analysis revealed a profound effect on sarcomere content and filament orientation when iPSC-derived cardiomyocytes were cultured on structured, line-shaped surfaces. Similarly, prolonged cultivation time had a beneficial effect on the structural maturation, leading to a more adult-like phenotype. Automatic evaluation of the sarcomere filaments by machine learning validated our data. Moreover, we successfully transferred this approach to skeletal muscle cells, showing an improved sarcomere formation cells over different differentiation periods. Overall, our image-based workflow can be used as a straight-forward tool to quantitatively estimate the structural maturation of contractile cells. As such, it can support the establishment of novel differentiation protocols to enhance sarcomere formation and maturity.
Related collections
Most cited references64
- Record: found
- Abstract: found
- Article: not found
Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors.

- Record: found
- Abstract: found
- Article: found
ThunderSTORM: a comprehensive ImageJ plug-in for PALM and STORM data analysis and super-resolution imaging
- Record: found
- Abstract: found
- Article: not found