- Record: found
- Abstract: found
- Article: found
Investigating Evolutionary Rate Variation in Bacteria
Read this article at
Abstract
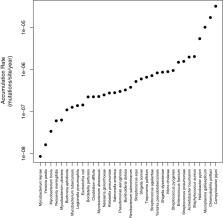
Rates of molecular evolution are known to vary between species and across all kingdoms of life. Here, we explore variation in the rate at which bacteria accumulate mutations (accumulation rates) in their natural environments over short periods of time. We have compiled estimates of the accumulation rate for over 34 species of bacteria, the majority of which are pathogens evolving either within an individual host or during outbreaks. Across species, we find that accumulation rates vary by over 3700-fold. We investigate whether accumulation rates are associated to a number potential correlates including genome size, GC content, measures of the natural selection and the time frame over which the accumulation rates were estimated. After controlling for phylogenetic non-independence, we find that the accumulation rate is not significantly correlated to any factor. Furthermore, contrary to previous results, we find that it is not impacted by the time frame of which the estimate was made. However, our study, with only 34 species, is likely to lack power to detect anything but large effects. We suggest that much of the rate variation may be explained by differences between species in the generation time in the wild.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Testing for phylogenetic signal in comparative data: behavioral traits are more labile.
- Record: found
- Abstract: found
- Article: not found
Time-dependent rates of molecular evolution.
- Record: found
- Abstract: found
- Article: not found