- Record: found
- Abstract: found
- Article: found
Amiodarone Enhances Anticonvulsive Effect of Oxcarbazepine and Pregabalin in the Mouse Maximal Electroshock Model
Read this article at
Abstract
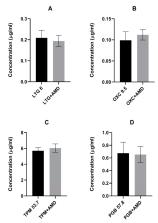
Accumulating experimental studies show that antiarrhythmic and antiepileptic drugs share some molecular mechanisms of action and can interact with each other. In this study, the influence of amiodarone (a class III antiarrhythmic drug) on the antiseizure action of four second-generation antiepileptic drugs was evaluated in the maximal electroshock model in mice. Amiodarone, although ineffective in the electroconvulsive threshold test, significantly potentiated the antielectroshock activity of oxcarbazepine and pregabalin. Amiodarone, given alone or in combination with oxcarbazepine, lamotrigine, or topiramate, significantly disturbed long-term memory in the passive-avoidance task in mice. Brain concentrations of antiepileptic drugs were not affected by amiodarone. However, the brain concentration of amiodarone was significantly elevated by oxcarbazepine, topiramate, and pregabalin. Additionally, oxcarbazepine and pregabalin elevated the brain concentration of desethylamiodarone, the main metabolite of amiodarone. In conclusion, potentially beneficial action of amiodarone in epilepsy patients seems to be limited by neurotoxic effects of amiodarone. Although results of this study should still be confirmed in chronic protocols of treatment, special precautions are recommended in clinical conditions. Coadministration of amiodarone, even at low therapeutic doses, with antiepileptic drugs should be carefully monitored to exclude undesired effects related to accumulation of the antiarrhythmic drug and its main metabolite, desethylamiodarone.
Related collections
Most cited references37
- Record: found
- Abstract: not found
- Article: not found
A simplified method of evaluating dose-effect experiments.
- Record: found
- Abstract: found
- Article: not found
Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery.
- Record: found
- Abstract: found
- Article: not found