- Record: found
- Abstract: found
- Article: found
Correlation between Blood and CSF Compartment Cytokines and Chemokines in Subjects with Cryptococcal Meningitis
Read this article at
Abstract
Background
Though peripheral blood is a crucial sample to study immunology, it is unclear whether the immune environment in the peripheral vasculature correlates with that at the end-organ site of infection. Using cryptococcal meningitis as a model, we investigated the correlation between serum and cerebrospinal fluid biomarkers over time.
Methods
We analyzed the cerebrospinal fluid and serum of 160 subjects presenting with first episode cryptococcal meningitis for soluble cytokines and chemokines measured by Luminex assay. Specimens were collected at meningitis diagnosis, 1-week, and 2-week post cryptococcal diagnosis. We compared paired samples by Spearman's correlation and the p value was set at <0.01.
Results
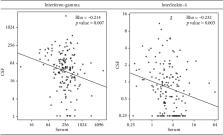
Of the 21 analytes tested at baseline, there was no correlation detected between nearly all analytes. A weak negative correlation was found between serum and cerebrospinal fluid levels of interferon-gamma (Rho = −0.214; p = .007) and interleukin-4 (Rho = −0.232; p = .003). There was no correlation at 1-week post cryptococcal diagnosis. However, at 2-week post cryptococcal diagnosis, there was a weak positive correlation of granulocyte-macrophage colony-stimulating factor levels (Rho = 0.25; p = .007) in serum and cerebrospinal fluid. No cytokine or chemokine showed consistent correlation overtime.
Conclusion
Based on our analysis of 21 biomarkers, serum and cerebrospinal fluid immune responses do not correlate. There appears to be a distinct immune environment in terms of soluble biomarkers in the vasculature versus end-organ site of infection. While this is a model of HIV-related cryptococcal meningitis, we postulate that assuming the blood compartment is representative of the immune function at the end-organ site of infection may not be appropriate.
Related collections
Most cited references13

- Record: found
- Abstract: found
- Article: found
Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Record: found
- Abstract: found
- Article: not found
IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans.
- Record: found
- Abstract: found
- Article: not found