- Record: found
- Abstract: found
- Article: not found
Pharmacological Blockade of ASCT2-dependent Glutamine Transport Leads To Anti-tumor Efficacy in Preclinical Models
Abstract
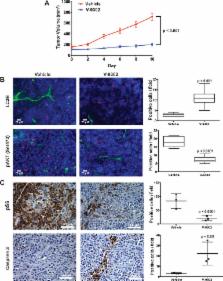
The unique metabolic demands of cancer cells underscore potentially fruitful opportunities for drug discovery in the era of precision medicine. However, therapeutic targeting of cancer metabolism has led to surprisingly few new drugs to date. The neutral amino acid glutamine serves as a key intermediate in numerous metabolic processes leveraged by cancer cells including biosynthesis, cell signaling, and oxidative protection. Herein, we report the preclinical development of V-9302, a competitive small molecule antagonist of transmembrane glutamine flux, that selectively and potently targets the amino acid transporter ASCT2 ( SLC1A5). Pharmacological blockade of ASCT2 with V-9302 resulted in attenuated cancer cell growth and proliferation, increased cell death, and increased oxidative stress, which collectively, contributed to anti-tumor responses in vitro and in vivo. Representing a new class of targeted therapy, this is the first study to demonstrate the utility of a pharmacological inhibitor of glutamine transport in oncology, laying a framework for paradigm-shifting therapies targeting cancer cell metabolism.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Bidirectional transport of amino acids regulates mTOR and autophagy.
- Record: found
- Abstract: found
- Article: found
Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer.
- Record: found
- Abstract: found
- Article: not found
