- Record: found
- Abstract: found
- Article: found
Pharmacological effects of recombinant human tissue kallikrein on bradykinin B 2 receptors
Read this article at
Abstract
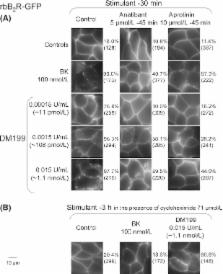
Tissue kallikrein (KLK-1), a serine protease, initiates the release of bradykinin (BK)-related peptides from low-molecular weight kininogen. KLK-1 and the BK B 2 receptor (B 2R) mediate beneficial effects on the progression of type 2 diabetes and renal disease, but the precise role of KLK-1 independent of its kinin-forming activity remains unclear. We used DM199, a recombinant form of human KLK-1, along with the isolated human umbilical vein, a robust bioassay of the B 2R, to address the previous claims that KLK-1 directly binds to and activates the human B 2R, with possible receptor cleavage. DM199 (1–10 nmol/L) contracted the isolated vein via the B 2R, but in a tachyphylactic, kinin-dependent manner, without desensitization of the tissue to exogenously added BK. In binding experiments with recombinant N-terminally tagged myc-B 2Rs expressed in HEK 293a cells, DM199 displaced [ 3H]BK binding from the rabbit myc-B 2R, but not from the human or rat myc-B 2Rs. No evidence of myc-B 2R degradation by immunoblot analysis was apparent following treatment of these 3 myc-B 2R constructs with DM199 (30 min, ≤10 nmol/L). In HEK 293 cells stably expressing rabbit B 2R-GFP, DM199 (11–108 pmol/L) elicited signaling-dependent endocytosis and reexpression, while a higher concentration (1.1 nmol/L) induced a partially irreversible endocytosis of the construct (microscopy), paralleled by the appearance of free GFP in cells (immunoblotting, indicative of incomplete receptor down-regulation). The pharmacology of DM199 at relevant concentrations (<10 nmol/L) is essentially based on the activity of locally generated kinins. Binding to and mild down-regulation of the B 2R is possibly a species-dependent idiosyncratic response to DM199.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Attenuation of green fluorescent protein half-life in mammalian cells.
- Record: found
- Abstract: found
- Article: not found
Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury.
- Record: found
- Abstract: found
- Article: not found
