- Record: found
- Abstract: found
- Article: found
Sfrp2 regulates the WNT/β-catenin pathway to slow the development of aldosterone-producing adenoma
Read this article at
Abstract
Background
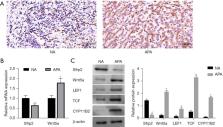
To explore a new drug therapy for aldosterone-producing adenoma (APA), and investigate whether Sfrp2 (secreted frizzled-related protein 2) can influence the development of adrenal APA by regulating the WNT/β-catenin pathway.
Methods
Tissue samples from APA patients were collected to detect the expression of Sfrp2 and β-catenin in APA. NCI-H295R cells were cultured with WNT/β-catenin pathway inhibitors to detect cell proliferation and aldosterone secretion. Then, the expression of Sfrp2 was altered to determine the effect of Sfrp2 expression on WNT/β-catenin pathway activity and aldosterone adenocarcinoma cells. Finally, a mouse APA model was established, and the mice were intravenously injected with WNT/β-catenin pathway inhibitors or transfected with the Sfrp2 gene. The activity of the WNT/β-catenin pathway, blood pressure, aldosterone secretion, and cell growth in the mice were then observed.
Results
β-catenin was overexpressed in APA tissues, while Sfrp2 was underexpressed. Sfrp2 can negatively regulate β-catenin expression and control the activity of the WNT/β-catenin pathway. Increased Sfrp2 expression inhibited the activity of the WNT/β-catenin pathway, which suppressed aldosterone secretion and APA cell proliferation. The in vivo experiments also demonstrated that inhibition of WNT/β-catenin pathway activity in mice reduced the arterial pressure and aldosterone concentration. The increased expression of Sfrp2 can inhibit the WNT/β-catenin pathway in mice, and can also reduce arterial pressure and APA tissue growth.
Conclusions
Sfrp2 can inhibit the WNT/β-catenin signaling pathway by suppressing the expression of β-catenin, thus controlling the concentration of aldosterone and hindering APA development. This study provides a novel therapeutic target for the treatment of APA and a new direction for future research.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities.
- Record: found
- Abstract: found
- Article: not found
Wnt/β-catenin signaling and disease.
- Record: found
- Abstract: found
- Article: not found