- Record: found
- Abstract: found
- Article: found
Morphometric and Microstructural Changes During Murine Retinal Development Characterized Using In Vivo Optical Coherence Tomography
Read this article at
Abstract
Purpose
The purpose of this study was to develop an in vivo optical coherence tomography (OCT) system capable of imaging the developing mouse retina and its associated morphometric and microstructural changes.
Methods
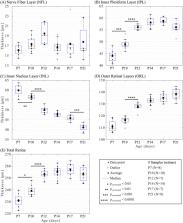
Thirty-four wild-type mice (129S1/SvlmJ) were anesthetized and imaged between postnatal (P) day 7 and P21. OCT instrumentation was developed to optimize signal intensity and image quality. Semi-automatic segmentation tools were developed to quantify the retinal thickness of the nerve fiber layer (NFL), inner plexiform layer (IPL), inner nuclear layer (INL), and the outer retinal layers (ORL), in addition to the total retina. The retinal maturation was characterized by comparing layer thicknesses between consecutive time points.
Results
From P7 to P10, the IPL increased significantly, consistent with retinal synaptogenesis. From P10 to P12, the IPL and ORL also increased, which is coherent with synaptic connectivity and photoreceptor maturation. In contrast, during these periods, the INL decreased significantly, consistent with cellular densification and selective apoptotic “pruning” of the tissue during nuclear migration. Thereafter from P12 to P21, the INL continued to thin (significantly from P17 to P21) whereas the other layers remained unchanged. No time-dependent changes were observed in the NFL. Overall, changes in the total retina were attributed to those in the IPL, INL, and ORL. Regions of the retina adjacent to the optic nerve head were thinner than distal regions during maturation.
Related collections
Most cited references92
- Record: found
- Abstract: found
- Article: not found
Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN•OCT consensus.
- Record: found
- Abstract: found
- Article: not found