- Record: found
- Abstract: found
- Article: found
Tonic inhibition of the chloride/proton antiporter ClC-7 by PI(3,5)P2 is crucial for lysosomal pH maintenance
Read this article at
Abstract
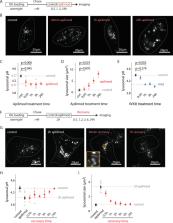
The acidic luminal pH of lysosomes, maintained within a narrow range, is essential for proper degrative function of the organelle and is generated by the action of a V-type H + ATPase, but other pathways for ion movement are required to dissipate the voltage generated by this process. ClC-7, a Cl -/H + antiporter responsible for lysosomal Cl - permeability, is a candidate to contribute to the acidification process as part of this ‘counterion pathway’ The signaling lipid PI(3,5)P2 modulates lysosomal dynamics, including by regulating lysosomal ion channels, raising the possibility that it could contribute to lysosomal pH regulation. Here, we demonstrate that depleting PI(3,5)P2 by inhibiting the kinase PIKfyve causes lysosomal hyperacidification, primarily via an effect on ClC-7. We further show that PI(3,5)P2 directly inhibits ClC-7 transport and that this inhibition is eliminated in a disease-causing gain-of-function ClC-7 mutation. Together, these observations suggest an intimate role for ClC-7 in lysosomal pH regulation.
Related collections
Most cited references56
- Record: found
- Abstract: not found
- Article: not found
A Threshold Selection Method from Gray-Level Histograms
- Record: found
- Abstract: found
- Article: not found
Genome-scale CRISPR-Cas9 knockout screening in human cells.
- Record: found
- Abstract: found
- Article: not found