- Record: found
- Abstract: found
- Article: found
Extracellular gamma-synuclein promotes tumor cell motility by activating β1 integrin-focal adhesion kinase signaling pathway and increasing matrix metalloproteinase-24, -2 protein secretion
Read this article at
Abstract
Background
Increasing evidence reveals a significant correlation between gamma-synuclein (SNCG) level and tumor invasion and metastasis in various human cancers. Our previous investigation showed that SNCG could secrete into extracellular environment and promoted tumor cell motility, but the mechanism is unknown.
Methods
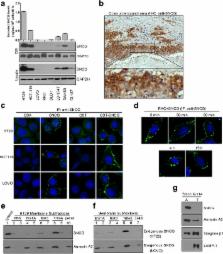
The membrane binding ability of SNCG was characterized by immunohistochemical staining, immunofluorescence staining and fractionation of colorectal cancer (CRC) cell membrane. Association between SNCG and β1 integrin was validated by coimmunoprecipitation and far Western blot. After inhibition of β1 integrin and focal adhesion kinase (FAK), effect of SNCG on cell motility was measured by transwell chamber assays and changes of protein levels were detected by Western blot. Association between SNCG and activated β1 integrin levels in human CRC tissues was determined by Spearman’s rank correlation analysis. Secreted proteins in conditioned medium (CM) were screened by antibody array.
Results
Extracellular SNCG bound β1 integrin on CRC cell membrane and increased levels of activated β1 integrin and FAK. Correspondingly, SNCG-enhanced cell motility was counteracted by knockdown or inhibition of β1 integrin or FAK. Further study revealed that high SNCG level indicated poor outcome and SNCG levels positively correlated with those of activated β1 integrin and phospho-FAK (Tyr 397) in human CRC tissues. Additionally, extracellular SNCG promoted secretion of fibronectin (FN), vitronectin (VN), matrix metalloproteinase (MMP)-2, and MMP-24 from HCT116 cells. Protease activity of MMP-2 in the CM of HCT116 cells was increased by treatment with SNCG, which was abolished by inhibiting β1 integrin.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
How matrix metalloproteinases regulate cell behavior.
- Record: found
- Abstract: found
- Article: not found
A matrix metalloproteinase expressed on the surface of invasive tumour cells.
- Record: found
- Abstract: found
- Article: not found