- Record: found
- Abstract: found
- Article: found
Protection of the transplant kidney during cold perfusion with doxycycline: proteomic analysis in a rat model
Read this article at
Abstract
Background
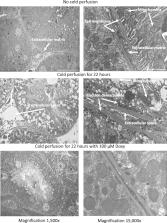
It has been previously shown that doxycycline (Doxy) protects the kidney from preservation injury by inhibition of matrix metalloproteinase. However, the precise molecular mechanism involved in this protection from injury is not known. We used a pharmaco-proteomics approach to identify potential molecular targets associated with kidney preservation injury.
Methods
Rat kidneys were cold perfused with or without doxycycline (Doxy) for 22 h. Kidneys perfusates were analyzed for the presence of injury markers such as lactate dehydrogenase (LDH), and neutrophil-gelatinase associated lipocalin (NGAL). Proteins extracted from kidney tissue were analyzed by 2-dimensional gel electrophoresis. Proteins of interest were identified by mass spectrometry.
Results
Triosephosphate isomerase, PGM, dihydropteridine reductase-2, pyridine nucleotide-disulfide oxidoreductase, phosphotriesterase-related protein, and aminoacylase-1A were not affected by cold perfusion. Perfusion with Doxy increased their levels. N(G),N(G)-dimethylarginine dimethylaminohydrolase and phosphoglycerate kinase 1 were decreased after cold perfusion. Perfusion with Doxy led to an increase in their levels.
Related collections
Most cited references31

- Record: found
- Abstract: found
- Article: found
Fast and Accurate Protein False Discovery Rates on Large-Scale Proteomics Data Sets with Percolator 3.0

- Record: found
- Abstract: found
- Article: found
Doxycycline Alters Metabolism and Proliferation of Human Cell Lines
- Record: found
- Abstract: found
- Article: not found