- Record: found
- Abstract: found
- Article: found
Effect of Electroacupuncture on Reuptake of Serotonin via miRNA-16 Expression in a Rat Model of Depression
Read this article at
Abstract
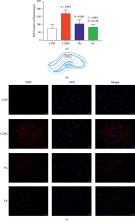
The current study aimed to investigate the effects and mechanisms of electroacupuncture (EA) treatment applied to Bai hui (GV20) and Yin tang (GV29) acupoints (1 mA, 2 Hz, continuous wave, 20 minutes) for 28 days in a rat model of chronic unpredictable mild stress (CUMS) on reuptake of serotonin (5-hydroxytryptamine (5-HT)) and miRNA-16 levels in the hippocampus and serum. Rats were housed in individual cages, and CUMS was used to establish a rat model of depression. After EA treatment for 4 weeks, behavioral changes and indices including 5-HT transporter (SERT), 5-HT, and miRNA-16 levels in the hippocampus and serum were examined. The EA treatment significantly improved base levels of sucrose preference and exploratory behavior and significantly decreased SERT protein and mRNA expression in the hippocampus of depressed rats. Significantly increased 5-HT levels were observed, and miRNA-16 levels were significantly decreased in the hippocampus and serum of depressed rats. In conclusion, the antidepressant effects of EA treatment may be affected via inhibition of 5-HT reuptake, upregulation of 5-HT levels, and inhibition of miRNA-16 expression in the hippocampus and serum.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Determining the role of microRNAs in psychiatric disorders.
- Record: found
- Abstract: not found
- Article: not found
Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression
- Record: found
- Abstract: found
- Article: not found