- Record: found
- Abstract: found
- Article: found
Effect of sperm DNA fragmentation on embryo development: clinical and biological aspects
Read this article at
Abstract
Objective
The aim of this study was to investigate the effect of sperm DNA fragmentation on fertilization rate, embryo development (blastulation rate), and pregnancy outcomes for ICSI cycles performed in a cohort of couples using donor eggs and to assess the remaining embryos that were not transferred or frozen for apoptotic markers.
Methods
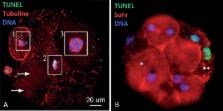
Eighty-two women (egg recipients) were included in the study (2016) were included in the study. The recipients' mean age was 41.8±5.1 y/o (36-49), while the egg donors' mean age was 30.8±2.1 y/o (27-33). Even though donor egg cycles with frozen sperm samples are performed regularly in our center, 35 cycles were done using fresh sperm samples. The mean age of the males involved in the procedure was 40.1±5.2 y/o. Fertilization, blastulation, and pregnancy rates were assessed. The patients were divided into two groups, TUNEL <15% and ≥15%. In arrested embryos, ICC was performed to detect cleaved caspase-3, survivin, TUNEL, and DNA. The Student's t-test was used in between-group comparisons. The Mann-Whitney U-test was used to assess homogeneity. Pearson's correlation coefficient was also calculated. p<0.05 was considered statistically significant.
Results
This study showed that there is a negative correlation (R=-0.5) between DNA fragmentation and blastulation rate. High levels of DNA fragmentation were associated with low blastulation and pregnancy rates (per transfer); however, fertilization rate was not affected. Samples with higher levels of DNA fragmentation were associated with higher levels of DNA fragmentation in blastomeres without activating the apoptotic pathway (9.1% vs. 15.9%) ( p<0.05). Blastomeres from samples with high DNA fragmentation activated the apoptotic pathway in higher levels than samples with TUNEL <15% (16.4% vs. 21.9%) ( p<0.05).
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting.
- Record: found
- Abstract: found
- Article: not found
Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome.
- Record: found
- Abstract: found
- Article: not found