- Record: found
- Abstract: found
- Article: found
A novel mutation in COL1A2 leads to osteogenesis imperfecta/Ehlers-Danlos overlap syndrome with brachydactyly
Abstract
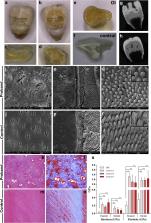
Osteogenesis imperfecta (OI) is mainly characterized by bone fragility and Ehlers-Danlos syndrome (EDS) by connective tissue defects. Mutations in COL1A1 or COL1A2 can lead to both syndromes. OI/EDS overlap syndrome is mostly caused by helical mutations near the amino-proteinase cleavage site of type I procollagen. In this study, we identified a Thai patient having OI type III, EDS, brachydactyly, and dentinogenesis imperfecta. His dentition showed delayed eruption, early exfoliation, and severe malocclusion. For the first time, ultrastructural analysis of the tooth affected with OI/EDS showed that the tooth had enamel inversion, bone-like dentin, loss of dentinal tubules, and reduction in hardness and elasticity, suggesting severe developmental disturbance. These severe dental defects have never been reported in OI or EDS. Exome sequencing identified a novel de novo heterozygous glycine substitution, c.3296G > A, p.Gly1099Glu, in exon 49 of COL1A2. Three patients with mutations in the exon 49 of COL1A2 were previously reported to have OI with brachydactyly and intracranial hemorrhage. Notably, two of these three patients did not show hyperextensible joints and hypermobile skin, while our patient at the age of 5 years had not developed intracranial hemorrhage. Here, we demonstrate that the novel glycine substitution in the carboxyl region of alpha2(I) collagen triple helix leads to OI/EDS with brachydactyly and severe tooth defects, expanding the genotypic and phenotypic spectra of OI/EDS overlap syndrome.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Genetic epidemiology, prevalence, and genotype-phenotype correlations in the Swedish population with osteogenesis imperfecta.
- Record: found
- Abstract: not found
- Article: not found
Mortality and Causes of Death in Patients With Osteogenesis Imperfecta: A Register-Based Nationwide Cohort Study
- Record: found
- Abstract: found
- Article: not found