- Record: found
- Abstract: found
- Article: found
Efficacy of a T Cell-Biased Adenovirus Vector as a Zika Virus Vaccine
Read this article at
Abstract
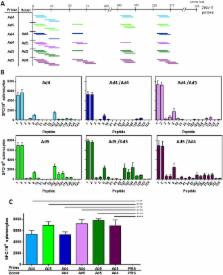
Zika virus (ZIKV) is a major public health concern due to the risk of congenital Zika syndrome in developing fetuses and Guillain-Barre syndrome in adults. Currently, there are no approved vaccines available to protect against infection. Adenoviruses are safe and highly immunogenic vaccine vectors capable of inducing lasting humoral and cellular immune responses. Here, we developed two Adenovirus (Ad) vectored Zika virus vaccines by inserting a ZIKV prM-E gene expression cassette into human Ad types 4 (Ad4-prM-E) and 5 (Ad5-prM-E). Immune correlates indicate that Ad5-prM-E vaccination induces both an anti-ZIKV antibody and T-cell responses whereas Ad4-prM-E vaccination only induces a T-cell response. In a highly lethal challenge in an interferon α/β receptor knockout mice, 80% of Ad5 vaccinated animals and 33% of Ad4 vaccinated animals survived a lethal ZIKV challenge, whereas no animals in the sham vaccinated group survived. In an infection model utilizing immunocompetent C57BL/6 mice that were immunized and then treated with a blocking anti-IFNAR-1 antibody immediately before ZIKV challenge, 100% of Ad4-prM-E and Ad5-prM-E vaccinated mice survived. This indicates that Ad4-prM-E vaccination is protective without the development of detectable anti-ZIKV antibodies. The protection seen in these highly lethal mouse models demonstrate the efficacy of Ad vectored vaccines for use against ZIKV.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys.
- Record: found
- Abstract: found
- Article: not found
Rapid development of a DNA vaccine for Zika virus.
- Record: found
- Abstract: found
- Article: not found