- Record: found
- Abstract: found
- Article: found
Excessive Accumulation of Ca 2 + in Mitochondria of Y522S-RYR1 Knock-in Mice: A Link Between Leak From the Sarcoplasmic Reticulum and Altered Redox State
Read this article at
Abstract
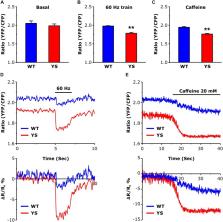
Mice (Y522S or YS), carrying a mutation of the sarcoplasmic reticulum (SR) Ca 2+ release channel of skeletal muscle fibers (ryanodine receptor type-1, RyR1) which causes Ca 2+ leak, are a widely accepted and intensively studied model for human malignant hyperthermia (MH) susceptibility. Since the involvement of reactive oxygen species (ROS) and of mitochondria in MH crisis has been previously debated, here we sought to determine Ca 2+ uptake in mitochondria and its possible link with ROS production in single fibers isolated from flexor digitorum brevis (FDB) of YS mice. We found that Ca 2+ concentration in the mitochondrial matrix, as detected with the ratiometric FRET-based 4mtD3cpv probe, was higher in YS than in wild-type (WT) fibers at rest and after Ca 2+ release from SR during repetitive electrical stimulation or caffeine administration. Also mitochondrial ROS production associated with contractile activity (detected with Mitosox probe) was much higher in YS fibers than in WT. Importantly, the inhibition of mitochondrial Ca 2+ uptake achieved by silencing MCU reduced ROS accumulation in the matrix and Ca 2+ release from SR. Finally, inhibition of mitochondrial ROS accumulation using Mitotempo reduced SR Ca 2+ release in YS fibers exposed to caffeine. The present results support the view that mitochondria take up larger amounts of Ca 2+ in YS than in WT fibers and that mitochondrial ROS production substantially contributes to the increased caffeine-sensitivity and to the enhanced Ca 2+ release from SR in YS fibers.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Mitochondria as sensors and regulators of calcium signalling.
- Record: found
- Abstract: found
- Article: not found
Regulation of mitochondrial dehydrogenases by calcium ions.
- Record: found
- Abstract: found
- Article: not found