- Record: found
- Abstract: found
- Article: found
Contractile Properties of Esophageal Striated Muscle: Comparison with Cardiac and Skeletal Muscles in Rats
Read this article at
Abstract
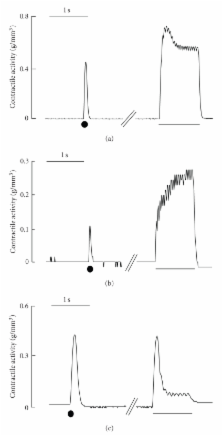
The external muscle layer of the mammalian esophagus consists of striated muscles. We investigated the contractile properties of esophageal striated muscle by comparison with those of skeletal and cardiac muscles. Electrical field stimulation with single pulses evoked twitch-like contractile responses in esophageal muscle, similar to those in skeletal muscle in duration and similar to those in cardiac muscle in amplitude. The contractions of esophageal muscle were not affected by an inhibitor of gap junctions. Contractile responses induced by high potassium or caffeine in esophageal muscle were analogous to those in skeletal muscle. High-frequency stimulation induced a transient summation of contractions followed by sustained contractions with amplitudes similar to those of twitch-like contractions, although a large summation was observed in skeletal muscle. The results demonstrate that esophageal muscle has properties similar but not identical to those of skeletal muscle and that some specific properties may be beneficial for esophageal peristalsis.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Structural and functional diversity of connexin genes in the mouse and human genome.
- Record: found
- Abstract: found
- Article: not found
Gap junctions: basic structure and function.
- Record: found
- Abstract: found
- Article: not found
