- Record: found
- Abstract: found
- Article: not found
Cofilin-mediated sorting and export of specific cargo from the Golgi apparatus in yeast
Read this article at
Abstract
Sorting of secretory cargo from the Golgi remains an elusive process. Previously a role was identified for cofilin and the Ca 2+ATPase SPCA1 in sorting of secretory cargo from the Golgi of mammalian cells. Now it is shown that the yeast orthologues cofilin and Pmr1 are also required for sorting of selective secretory cargo at the Golgi in yeast.
Abstract
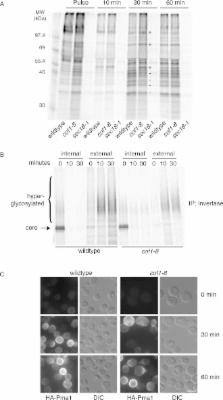
The mechanism of cargo sorting at the trans-Golgi network (TGN) for secretion is poorly understood. We previously reported the involvement of the actin-severing protein cofilin and the Ca 2+ ATPase secretory pathway calcium ATPase 1 (SPCA1) in the sorting of soluble secretory cargo at the TGN in mammalian cells. Now we report that cofilin in yeast is required for export of selective secretory cargo at the late Golgi membranes. In cofilin mutant ( cof1-8) cells, the cell wall protein Bgl2 was secreted at a reduced rate and retained in a late Golgi compartment, whereas the plasma membrane H + ATPase Pma1, which is transported in the same class of carriers, reached the cell surface. In addition, sorting of carboxypeptidase Y (CPY) to the vacuole was delayed, and CPY was secreted from cof1-8 cells. Loss of the yeast orthologue of SPCA1 (Pmr1) exhibited similar sorting defects and displayed synthetic sickness with cof1-8. In addition, overexpression of PMR1 restored Bgl2 secretion in cof1-8 cells. These findings highlight the conserved role of cofilin and SPCA1/Pmr1 in sorting of the soluble secretory proteins at the TGN/late Golgi membranes in eukaryotes.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
The mechanisms of vesicle budding and fusion.
- Record: found
- Abstract: found
- Article: not found
A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast
- Record: found
- Abstract: found
- Article: not found
